What Is The Purpose Of Reference Electrode . a reference electrode refers to an electrode that has an established electrode potential. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Reference electrodes used are preferably reversible type. reference electrodes should have stable and reproducible potential. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. In an electrochemical cell, the reference electrode can. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator.
from mungfali.com
in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. In an electrochemical cell, the reference electrode can. Reference electrodes used are preferably reversible type. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. reference electrodes should have stable and reproducible potential. a reference electrode refers to an electrode that has an established electrode potential.
Reference Electrode Diagram
What Is The Purpose Of Reference Electrode the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. reference electrodes should have stable and reproducible potential. Reference electrodes used are preferably reversible type. In an electrochemical cell, the reference electrode can. a reference electrode refers to an electrode that has an established electrode potential. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts What Is The Purpose Of Reference Electrode in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. the purpose of a reference electrode is to complete the electrical circuit. What Is The Purpose Of Reference Electrode.
From www.researchgate.net
Schematic description for reference electrode connection to MFC What Is The Purpose Of Reference Electrode reference electrodes should have stable and reproducible potential. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. In an electrochemical cell,. What Is The Purpose Of Reference Electrode.
From lab.dekresearch.com
How to Choose the Reference Electrode? What Is The Purpose Of Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Reference electrodes used are preferably reversible type. In an electrochemical cell, the reference electrode can. the. What Is The Purpose Of Reference Electrode.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes What Is The Purpose Of Reference Electrode in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. reference electrodes should have stable and reproducible potential. in potentiometry, those two electrodes are generally called the indicator electrode. What Is The Purpose Of Reference Electrode.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes What Is The Purpose Of Reference Electrode Reference electrodes used are preferably reversible type. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. a reference electrode refers to an electrode that has an established electrode potential. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the ideal. What Is The Purpose Of Reference Electrode.
From www.scribd.com
Reference Electrode PDF What Is The Purpose Of Reference Electrode in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. a reference electrode refers to an electrode that has an established electrode potential. in potentiometry, those two electrodes. What Is The Purpose Of Reference Electrode.
From www.hamiltoncompany.com
The pH Reference Electrode Process Analytics Hamilton Company What Is The Purpose Of Reference Electrode a reference electrode refers to an electrode that has an established electrode potential. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. Reference electrodes used are preferably reversible type.. What Is The Purpose Of Reference Electrode.
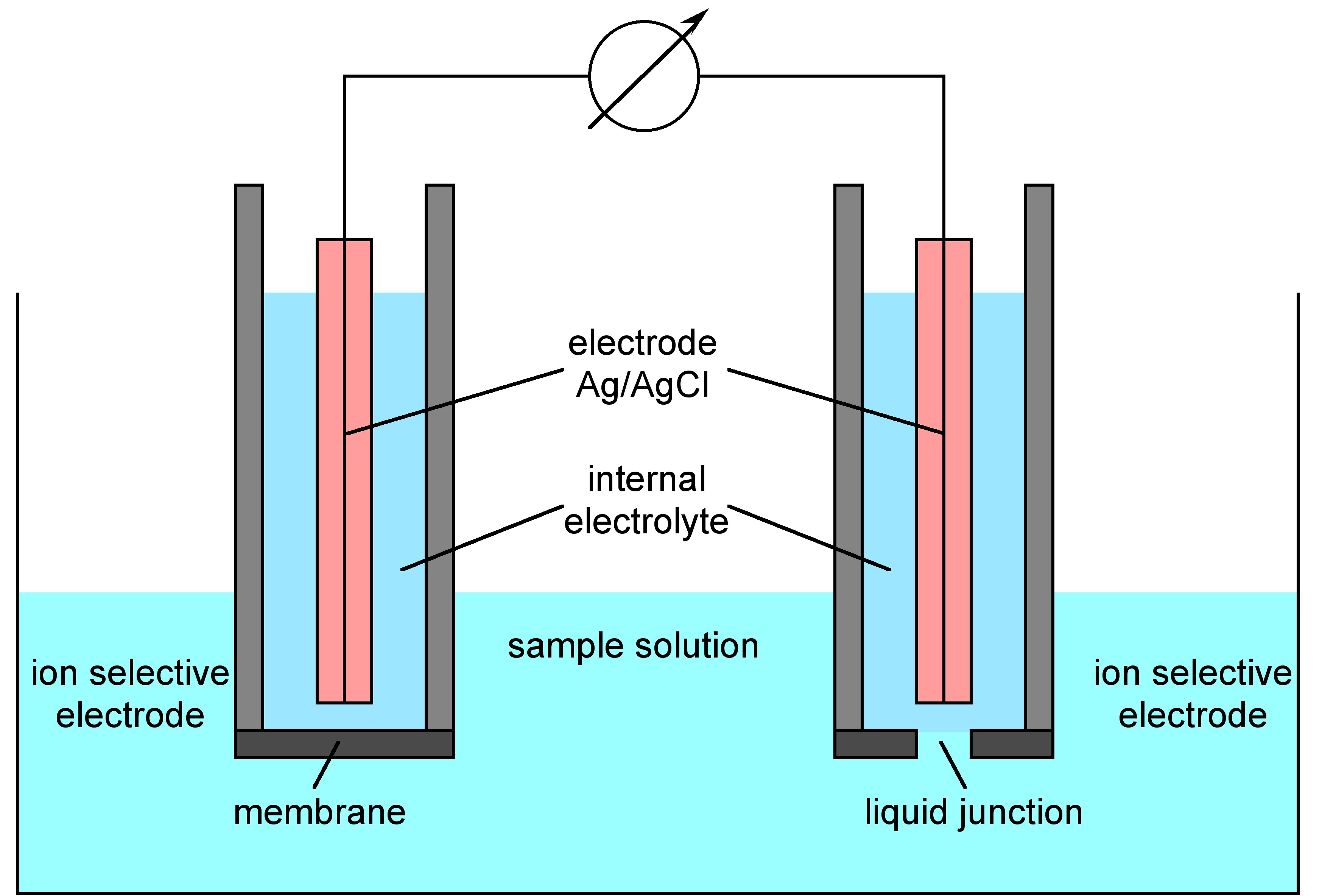
From mungfali.com
Reference Electrode Diagram What Is The Purpose Of Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. a reference electrode refers to an electrode that has an established electrode potential. the ideal reference electrode provides a stable, known potential. What Is The Purpose Of Reference Electrode.
From studylib.net
Reference electrodes and their usage What Is The Purpose Of Reference Electrode In an electrochemical cell, the reference electrode can. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. the purpose of a reference electrode is to complete the electrical circuit and provide a stable. What Is The Purpose Of Reference Electrode.
From www.researchgate.net
Schematic diagrams of reference electrodes (REs) used. On the left side What Is The Purpose Of Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. Reference electrodes used are preferably reversible type. a reference electrode refers to. What Is The Purpose Of Reference Electrode.
From www.gamry.com
Reference Electrodes Saturated CalomelSilver ChlorideMercury Oxide What Is The Purpose Of Reference Electrode reference electrodes should have stable and reproducible potential. the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. the purpose of the reference electrode is to complete the electrical circuit necessary for. What Is The Purpose Of Reference Electrode.
From mungfali.com
Reference Electrode Diagram What Is The Purpose Of Reference Electrode the purpose of a reference electrode is to complete the electrical circuit and provide a stable ground voltage to. In an electrochemical cell, the reference electrode can. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. a reference electrode refers to an electrode that has an established electrode. What Is The Purpose Of Reference Electrode.
From www.researchgate.net
What kind of reference electrode can be used in ionic liquids aqueous What Is The Purpose Of Reference Electrode reference electrodes should have stable and reproducible potential. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. In an electrochemical cell, the reference electrode can. the purpose of. What Is The Purpose Of Reference Electrode.
From anthropology.iresearchnet.com
Tools Measurement & Analysis Instruments AG Ag ion electrode micro What Is The Purpose Of Reference Electrode reference electrodes should have stable and reproducible potential. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. a reference electrode refers to an electrode that has an established electrode potential. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. In an. What Is The Purpose Of Reference Electrode.
From www.researchgate.net
"Open" and "close" designs of the reference electrode compartment of What Is The Purpose Of Reference Electrode the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. Reference electrodes used are preferably reversible type. a reference electrode refers to an electrode that has an established electrode potential. the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell. What Is The Purpose Of Reference Electrode.
From askfilo.com
29. Define reference electrode. Write the functions of salt bridge. Draw What Is The Purpose Of Reference Electrode reference electrodes should have stable and reproducible potential. a reference electrode refers to an electrode that has an established electrode potential. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. in this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen. What Is The Purpose Of Reference Electrode.
From www.askdifference.com
Indicator Electrode vs. Reference Electrode — What’s the Difference? What Is The Purpose Of Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. in potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Reference electrodes used are preferably reversible type. the purpose of a reference electrode is to complete. What Is The Purpose Of Reference Electrode.
From www.slideserve.com
PPT Uses of Reference Electrodes for various purposes PowerPoint What Is The Purpose Of Reference Electrode the ideal reference electrode provides a stable, known potential so that we can attribute any change in e cell to the analyte’s effect on the indicator. Reference electrodes used are preferably reversible type. the purpose of the reference electrode is to complete the electrical circuit necessary for an electrochemical measurement by. in potentiometry, those two electrodes are. What Is The Purpose Of Reference Electrode.